- Home
- >
- Industries
- >
- Veterinary
Global Veterinary and Animal Nutrition Regulatory Services

Full-Spectrum Veterinary Life Science
Services: From Concept to Market
At Artixio, we empower veterinary and animal nutrition (feed) businesses to bring groundbreaking solutions to market globally. Our expertise stretches across the entire product journey, from initial research and development to successful product launch and beyond. We work with our clients at every step, providing the guidance and support they need to overcome regulatory challenges and achieve their business goals.
Services
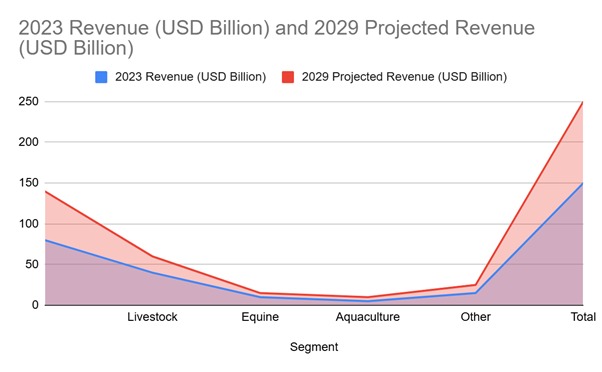
Projected Growth of Global Veterinary Services Market by Segment
Detailed Steps for Veterinary Registration Pathway
Phase 1: Pre-Registration Preparation
| Step | Description | Key Activities |
|---|---|---|
| 1. Product Development & Characterization | Characterization: Define product composition, manufacturing, and quality control. | Formulation development, analytical method development, pre-formulation studies. |
| 2. Safety & Efficacy Studies | Demonstrate product safety and effectiveness in target animals. | Target animal safety studies (toxicity, etc.), efficacy studies, residue studies (if applicable). |
| 3. Environmental Impact Assessment | Evaluate potential environmental effects. | Assessment of product’s impact on water, soil, and wildlife. |
| 4. Manufacturing Process Development & Validation | Optimize and validate manufacturing for consistent quality. | Scale-up, process validation, quality control system implementation. |
| 5. Regulatory Strategy & Consultation | Determine the regulatory pathway and consult with the agency. | Regulatory Feasibility Analysis of Active Ingredients, Pre-submission meetings, data gap analysis. |
Phase 2: Application Submission & Review
| Step | Description | Key Activities |
|---|---|---|
| 6. Dossier Preparation | Compile all required data and documents. | Assembling safety and efficacy reports, manufacturing information, labeling. |
| 7. Application Submission | Submit the complete dossier to the regulatory authority. | Filing the application form, paying fees. |
| 8. Preliminary Review | Agency screens the application for completeness. | Checking for required documents and format. |
| 9. Technical Review | Experts evaluate the safety, efficacy, and quality data. | Review of study reports, manufacturing process, labeling. |
| 10. Requests for Information (RFI) | Agency requests clarification or additional data. | Responding to RFIs promptly and thoroughly. |
| 11. Inspections (if applicable) | Agency inspects manufacturing facilities. | Verification of GMP compliance. |
Phase 3: Approval
| Step | Description | Key Activities |
|---|---|---|
| 12. Approval/Authorization | Agency grants marketing authorization if requirements are met. | Review of all data and granting of approval. |
Phase 4: Post-Market Surveillance
| Step | Description | Key Activities |
|---|---|---|
| 13. Post-Market Surveillance | Monitor product safety and effectiveness after approval. | Adverse event reporting, periodic safety reports. |
| 14. Market Entry & Commercialization | Launch and market the product. | Marketing, sales, and distribution. |
Stay Ahead of the Curve: Key Trends Shaping the Veterinary Industry
The Convergence where the Human and Animal Health meet:
Interesting Fact:
What it means to Veterinary Industries:
The Growing Importance of Sustainability in Veterinary Life Sciences:
Interesting Fact:
What it means to Industry Leaders:
FAQs
1. How does Artixio help maximize ROI for veterinary product development?
2. What are the key elements of a successful regulatory strategy?
3. How does Artixio accelerate the time to market for our veterinary products?
4. How does Artixio help you prioritize veterinary compliance filings?
5. How can I learn more about Artixio's veterinary life science services?
Get in touch with us today at info@artixio.com for a free consultation to discuss your specific needs.
Contact us at info@artixio.com for a free consultation to discuss your veterinary product registration needs.
Why Choose Artixio?

Global Regulatory Intelligence Driven Solutions

200+ veterinary products reviews, approvals and lifecycle management

Global veterinary expertise: 70+ markets, 15-35 years.

ISO 9001:2015: Quality in veterinary service.

Your veterinary regulatory needs, our solutions: Flexible and customer-focused
Regulatory Expertise Across
Multiple Countries












Insights from Artixio - Tips & Articles

Pharma Manufacturing Booms in
Hildalgo had good pharmaceutical infrastructure and supply chain capabilities, but with no foreign investment...

UK Releases Amendment &
In January 2025, MHRA UK released guidance on implementation of amendments to Medical Devices...

Artixio Announces Grant for
HYDERABAD, India - March 27, 2024 - Artixio, a leading compliance and commercial consulting...






