- Home
- >
- Services
Global Regulatory Compliance Services
Artixio’s global services ensure your innovations are safe and effective by ensuring compliance with global health authorities guidelines and regulations. Our regulatory intelligence, tech assisted, services are designed to support your journey from development to commercial launch to post market safety and regulatory compliance delivering seamless experience.

Our Services
Our Services
Publishing &
Submission Services

Quality Services

Testing Services
Publishing &
Submission Services

Market Access

MDR Gap Assessment

Digital Marketing
End-to-End Global Regulatory Services
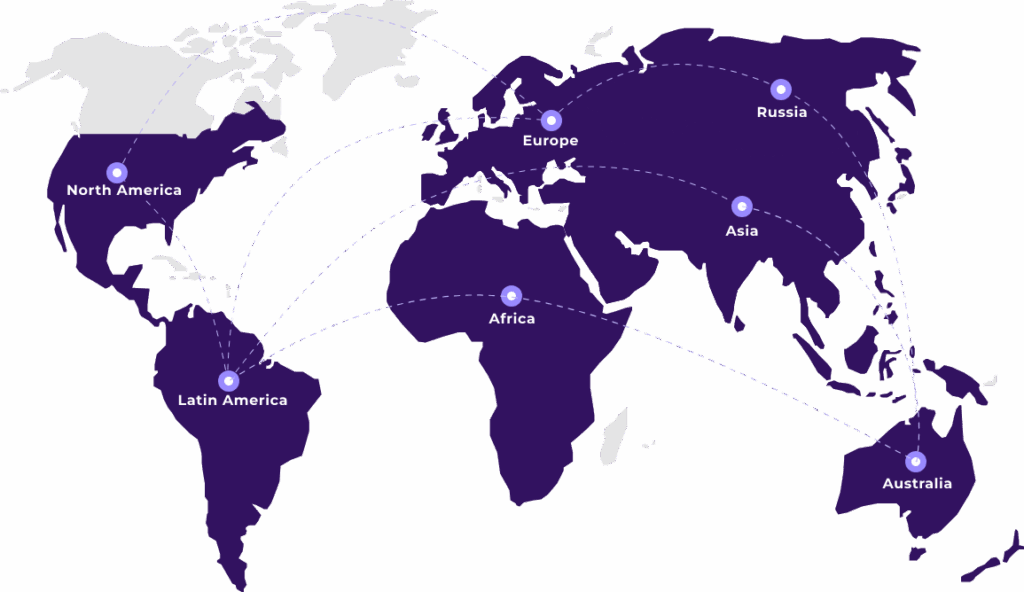
Supporting Life Sciences companies across 120+ countries — from product registration to post-market compliance, we handle the tough regulatory work so you don’t have to.

Your Global Partner in Regulatory Success
At Artixio, we handle regulatory affairs for the life sciences industry globally. Our team brings global expertise and local insight to help you meet compliance needs across Pharmaceuticals, MedTech, Cosmetics, Nutrition, Biologics, and Veterinary products. Whether you’re expanding into new markets or keeping up with ongoing requirements, we make the process easier with practical, end-to-end support.
Why Companies Choose Artixio
- Strong regional expertise across 120+ markets
- 15–35 years of experience across our leadership team
- Certified experts with global and local insight
- Trusted by 100+ clients across the world
- ISO 9001:2015 certified for quality management
- Direct communication with Health Authorities
- Deep country knowledge backed by global perspective
- Full support from registration to post-launch
Our Industry-Specific Solutions
Our Regulatory Solutions by Country
Blogs

Pharma Manufacturing Booms in
Hildalgo had good pharmaceutical infrastructure and supply chain capabilities, but with no foreign investment...
May 2, 2025

UK Releases Amendment &
In January 2025, MHRA UK released guidance on implementation of amendments to Medical Devices...
April 23, 2025

Artixio Announces Grant for
HYDERABAD, India - March 27, 2024 - Artixio, a leading compliance and commercial consulting...
April 16, 2025