The Modernization of Cosmetics Regulation Act (MoCRA), which was signed into law on December 29, 2022, is a significant milestone in the regulation of cosmetics in the US. The MoCRA is an important enhancement of the FDA’s power in overseeing cosmetics and surpasses the scope of control granted by the Federal Food, Drug, and Cosmetic (FD&C) Act established in 1938. The MoCRA is a bipartisan effort aimed at enhancing the FDA’s oversight and ensuring the safety of cosmetic products for consumers.
The Modernization of Cosmetics Regulation Act (MoCRA) grants the US FDA enhanced jurisdiction and establishes fresh obligations for the cosmetic sector in the United States. The FDA is now authorized to both acquire cosmetic records, and can enforce mandatory recalls if it determines that specific cosmetic products may have detrimental impacts on consumers’ health.
Applicability of Modernization of Cosmetics Regulation Act (MoCRA)
MoCRA is an extensive regulation that is applicable to all companies involved in the production, importation, marketing, and distribution of cosmetics, as well as to all categories of cosmetic products. The legislation also covers the constituents utilized in cosmetics products and the manufacturing processes employed to create them.
Regulatory Obligations for cosmetic manufacturers under MoCRA
In the cosmetics industry, stakeholders are now subjected to below regulatory requirements –
- Register their facilities, which must be renewed every two years according to FDA regulations. Small businesses may be exempt from this requirement.
- For products that are introduced to the market after December 29, 2023, the responsible person must provide a product listing within 120 days of marketing these products in interstate commerce. This listing must be updated annually and is crucial for the FDA to track the products and ingredients used by manufacturers, ensuring their safety and effectiveness, as well as compliance with Good Manufacturing Practices (GMP).
- A Responsible Person must be designated to submit reports on serious adverse events within 15 working days using Form 3500A.
- Pharmacovigilance (PV) procedures must be established to receive, assess, and address adverse event reports related to their products. The pharmacovigilance system should also include procedures for collecting, analyzing, and communicating adverse event data to the FDA. Each serious adverse event report should be retained for a period of six years, with exceptions given to small businesses. It is necessary for these reports to provide comprehensive details about the events, including the specific date, time, location, severity, and any actions taken to resolve the situation.
- To support claims of product safety, MoCRA requires the availability of safety data, which can be obtained from various sources like scientific studies and consumer reports.
- Manufacturing facilities that produce cosmetic products must adhere to the Good Manufacturing Practice (GMP) requirements. This includes documenting the manufacturing process and subjecting the facilities to regular inspections and monitoring by the FDA.
- Every cosmetic product being marketed must be registered with the FDA by a responsible individual. This registration should include information about the product’s ingredients, and any necessary updates must be provided annually.
- The responsible person is responsible for maintaining records that substantiate the safety claims of their cosmetic products. Manufacturers can utilize existing safety data to prove the safety of their products, but it is important to ensure that all safety data is obtained through scientifically valid methods. Animal testing is not obligatory for the marketing of cosmetic products.
- Regulations are in place for fragrance allergen labeling.
- Standardized testing methods have been established to detect and identify asbestos in cosmetic products that contain talc.
Implementation of Modernization of Cosmetics Regulation Act (MoCRA)
In the past, registration of cosmetic products and manufacturing facilities for cosmetics was optional. However, it is now mandatory to register cosmetics, and as a result, the US FDA has stopped accepting electronic or paper submissions since March 2023.
To prepare for the implementation of new regulations under MOCRA, the US FDA has been issuing drafts of guidelines that cover the registration of cosmetic product facilities and products. The Agency has also released technical specifications for the implementation of Structured Product Labeling (SPL) and the validation procedures. The draft guideline for Good Manufacturing Practices in the cosmetic market in the USA is also to be finalized and released by US FDA.
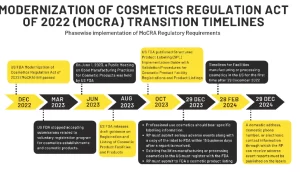
MoCRA Compliance transition timelines
The US FDA has given different transition timelines for different regulatory obligations. By 29th December 2023, professional use cosmetics should bear specific labelling information; RP must submit serious adverse events along with a copy of the label to FDA within 15 business days after a report is received; existing facilities manufacturing or processing cosmetics in the US must register with the FDA; the Responsible Person must submit to FDA a cosmetic product listing.
By 29 December 2024, a domestic address, domestic phone number, or electronic contact information through which the RP can receive adverse event reports is required on product’s label before.
The facilities manufacturing or processing cosmetics in the US for the first time after 29 December 2022 have an additional 60 days to register and must register by February 28 2024.
If the products are first marketed after 29 December 2023, RP must submit a product listing within 120 days of marketing such products in interstate commerce followed by annual updates to such listing.
Exceptions to the regulatory requirements of the US FDA Modernization of Cosmetics Regulation Act (MoCRA)
MoCRA provides exemptions for specific small businesses from the requirements of GMP, registration, and product listing. However, these exemptions do not apply to manufacturers or facilities that produce or process certain cosmetic products:
- Products that regularly come into contact with the eye’s mucus membrane under normal conditions of use.
- Injected products.
- Internally used products.
- Products that change one’s appearance for more than 24 hours under normal conditions of use, and consumer removal is not considered part of these conditions.
Previously, registration of cosmetic products and manufacturing facilities was optional. However, registration has now become mandatory for cosmetics, and the US FDA has discontinued accepting both electronic and paper submissions since March 2023. Cosmetic manufacturers and importers are now required to comply with the new regulations. Certain exemptions also exist for products and facilities that are subject to requirements for drugs and devices.
MoCRA is a groundbreaking legislation that strengthens the FDA’s oversight of the cosmetics industry and ensures the safety of cosmetic products for consumers. Although MoCRA imposes new obligations on cosmetic manufacturers and distributors, the benefits to consumers are evident, as it guarantees the safety and effectiveness of the cosmetics they use daily.
Cosmetic manufacturers and importers now have the responsibility to comply with these new regulations. “Artixio has the ability to assist you with”
- US Agent Services for non-US Cosmetics manufacturers
- Cosmetic Products Facility Registration
- Cosmetic Product Listing
- Responsible Person for Cosmetics
- MoCRA Labeling Compliance
- Safety Substantiation
- SPL for the cosmetic submissions
- Cosmetic GMP compliance
- Adverse Event Reporting and Management
FAQs – Modernization of Cosmetics Regulation Act of 2022
Why Is This New Law So Significant?
It’s not just that it’s been more than 80 years since the passing of the FDCA, but also the fact that it only provided general guidance outside of one requirement, that being that finished products be safe when used in accordance with labeling. Modernization Cosmetics Regulation Act 2022 MoCRA aims to achieve that.
How Do I Know if Modernization of Cosmetics Regulation Act 2022 Applies to My Business?
The criteria for exemption from MoCRA is fairly straightforward. Generally, the requirements apply to all cosmetics manufacturers, packers, importers and distributors in the U.S., as well as those outside the country that market their products in the U.S.
What are the new authorities provided to FDA by MoCRA?
Modernization Cosmetics Regulation Act 2022 provides new authorities to FDA including: Records Access, Mandatory Recall Authority.
Adverse Event Reporting: A responsible person is required to report serious adverse events associated with the use of cosmetic products in the United States to FDA within 15 business days. The responsible person must include a copy of the label on or within the retail packaging of such cosmetic product1. If the responsible person receives medical or other information about the adverse event within 1 year of the initial report to FDA, they must submit this new information to FDA within 15 business days.
Mandatory Facility and Product Registration: MoCRA mandates that cosmetics manufacturers and processors must register their facilities and products.
Under the Modernization of Cosmetics Regulation Act of 2022 (MoCRA), there are several key details regarding Mandatory Facility and Product Registration:
Facility Registration: Owners and operators of U.S. and foreign-based facilities that manufacture and process cosmetics distributed in the U.S. are required to register their facilities with the FDA. The registration should include:
- The name of the owner/operator of the facility.
- The facility’s physical address and information for a contact person.
- The FDA Establishment Identifier (FEI) as the required facility registration number.
Product Listing: Manufacturers, packers, and distributors of cosmetics in the U.S. are required to submit listings of their cosmetics products (and ingredients) to the agency. The listing should include:
-A list of all brand names of the cosmetic products manufactured or processed at the facility.
-The responsible person for each cosmetic product.
-The product category or categories into which each product falls (based on the options listed in the Appendix A to the Final Guidance).
The FDA has developed a draft electronic submission portal (Cosmetics Direct) and paper forms (Forms FDA 5066 and 5067) to facilitate the registration and listing process. The agency strongly encourages electronic submissions to facilitate efficiency and timeliness of data submission and management. However, respondents that prefer to submit paper registrations and listings will still have the option to do so.
- Adherence to Cosmetics Good Manufacturing Practices (GMPs).
- Safety Substantiation: The Act includes new requirements such as documentation for safety substantiation.
- Labeling Changes: The Act also includes new requirements for labeling changes.
These new requirements aim to ensure the safety of cosmetic products that many consumers use daily using the Modernization Cosmetics Regulation Act 2022 MoCRA.
For more detailed information, you may want to refer to the official FDA resources or consult with Artixio expert.




