- Home
- >
- All Countries
- >
- Taiwan
Life Science Compliance Services in Taiwan
We provide overarching regulatory services across all life science products. Leverage Artixio’s strong regulatory compliance and solution services for smooth transition from product development to registration and market authorization. Partner with us for expedited regulatory approvals.
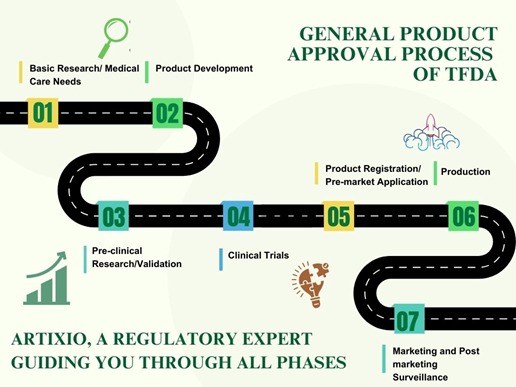
Regulatory Authorities in Taiwan
Initially it was the Bureau of Pharmaceutical Affairs, the Bureau of Food Safety, the Bureau of Food and Drug Analysis and the Bureau of Controlled Drugs. All these were consolidated on January 1, 2010, into the current TFDA. The goal of both the regulators is to ensure the safe and quality reach of the products to the consumers so as to safeguard the public as well as animal health.
What We Do at Artixio?
Where does our expertise come from?
Post-registration
Market entry services, Post market Surveillance, Product lifecycle management services, performance evaluation reports, Advertisement and promotional material review, Digital marketing and other ongoing support services.
Services through all phases
Registration
Pre-registration
Our Services



Why Artixio?










Industries We Serve in Taiwan
FAQs
What are some common challenges faced by foreign manufacturers in Taiwan?
What is the RTF procedure?
What is the function of TFDA?
What are the criteria for an application to undergo priority review under TFDA?
For an application to undergo priority review process, it must meet any 2 of the following criteria: i) It must be new chemical entity, combination, indication or must have a new administration route, ii) Must meet the unmet medical needs (in the treatment of serious conditions) and should have a significant clinical advance if approved, iii) Addresses an unmet medical need and is under the special national scientific research and development programs.
What is PIC/S and is TFDA a member of PIC/S?
Our Global Reach: Serving Life
Sciences Clients Worldwide

India

Singapore

Mexico

Brazil

Vietnam

Malaysia

Argentina

Colombia

Taiwan

China

European

Thailand

Indonesia

Philippines

USA

Japan

Qatar

South Korea
Insights from Artixio - Tips & Articles

Pharma Manufacturing Booms in
Hildalgo had good pharmaceutical infrastructure and supply chain capabilities, but with no foreign investment...

UK Releases Amendment &
In January 2025, MHRA UK released guidance on implementation of amendments to Medical Devices...

Artixio Announces Grant for
HYDERABAD, India - March 27, 2024 - Artixio, a leading compliance and commercial consulting...






