Pharmacovigilance (PV) is one of the most important parts of the drug product process. PV deals with the detection, assessment and revision of the adverse effects associated with the drugs.
However, after deep and detailed study analysis some adverse effects are only understood after its administration to the population for long term. So, for this it is necessary to keep track of the drug’s reaction in the population after its administration. So, for this we can make use of documents such as ICSR, PSMF, etc.
What is Pharmacovigilance System Master File (PSMF)?
Pharmacovigilance System Master File (PSMF) is a document required by the EU after the market approval of the drug which comprises of all the details of the pharmacovigilance aspect of the drug product.
If one desires to launch its drug product into the European market, he should apply for market authorization (MA) for its drug approval, after which the EMA which is the regulatory body of EU may ask for PSMF which should be submitted within 7 days or else your MA may be rejected.
Regulatory Basis of Pharmacovigilance System Master File (PSMF)
The legal framework mandating marketing authorization holders (MAHs) to maintain and provide a Pharmacovigilance System Master File (PSMF) finds its roots in
- Directive 2010/84/EU and
- Regulation (EU) No 1235/2010
- Directive 2001/83/EC
- Implementing Regulation (EU) No 520/2012.
This regulatory framework, supplemented by guidance in the Good Pharmacovigilance Practices (GVP) Module, sets the stage for comprehensive and standardized pharmacovigilance practices across the EU.
Importance of PSMF In Pharmacovigilance Regulations?
- PSMF is a document important for the safety of the drug.
- It helps to study and understand the safety of the drugs and adverse reactions associated with it and how to minimize them.
- It helps to achieve and fulfill the regulatory requirements.
- It is used as an evaluation document for the company’s pharmacovigilance parameter.
- It promotes patient safety.
- It is a healthy method of continuous improvement for drug safety.
PSMF Requirements:
- PSMF should follow Good Pharmacovigilance Practice (GVP) Module II requirements.
- The product for which PSMF is being submitted should be applicable to market authorization (MA).
- Details of the drug product if made any.
- The status of drug approval.
- It should be stated that how the PV system was implemented in the drug.
Structure & Content Of PSMF In Pharmacovigilance:
The structure of PSMF consists of three major parts namely cover page, main body, and annexes which are described below:
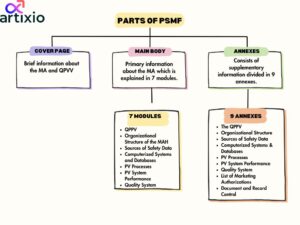
- Cover Page: It consists of a brief abstract of the MA and all the information about the qualified person for pharmacovigilance.
- Main Body: It comprises of primary information about the MA and the main body is divided into 7 modules which are:
QPPVOrganizational Structure of the MAHSources of Safety DataComputerized Systems and Databases
PV Processes
PV System Performance
Quality System
Each module comprises of detailed information.
- Annexes: This part includes any supplementary information that gives more detailed information about any of the modules from the main body. There are overall 9 Annexes which are:
The QPPVOrganizational StructureSources of Safety DataComputerized Systems & Databases
PV Processes
PV System Performance
Quality System
List of Marketing Authorizations
Document and Record Control
How To Prepare And Maintain A PSMF:
- To prepare a PSMF first understanding of all the regulatory requirements is necessary such as the GVP Module II, other international standards, etc.
- Then accurate and relevant information is to be collected about the pharmacovigilance of the drug and then accordingly the PSMF should be prepared in three sections, that is the cover page, main body and annexes followed by their subheadings.
- After that amend any changes if required and go to the next step for review and approval.
- For maintaining a PSMF train the staff accordingly and assign specific duties to each of the team member.
- Continuously keep updating your PSMF to the current PV data.
- Prepare and maintain PSMF in accordance with quality management practices.
Location For Registration Of PSMF:
While submission of the PSMF it should include a part called “Summary of the Pharmacovigilance System” and this summary should include the location where the PSMF is kept.
This summary is needed tp submit with the marketing authorization application.
The location for the EU PSMF should be where the QPVV operates or where all the PV activities take place.
The EU PSMF has a unique code assigned by the EMA’s Eudravigilance system which should be attached to the PSMF location.
Number Of PSMF Required:
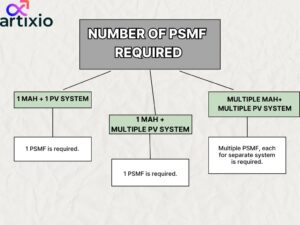
The number of PSMF are decided according to the situation; which is explained below:
- For one Marketing Authorization Holder (MAH) and one PV system one PSMF is required.
- For one Marketing Authorization Holder (MAH) and multiple PV system one PSMF is required.
- For multiple Marketing Authorization Holder (MAH) and multiple PV system multiple PSMF are required; for which each system should be explained in different PSMF.
Who should develop and maintain Pharmacovigilance System Master File (PSMF)?
The requirement for a PSMF extends to all medicinal products authorized in the EU, regardless of whether they underwent the centralized or decentralized procedure. However, there are specific considerations for different categories of medicinal products.
- Traditional Herbal Medicines: For products registered under the simplified registration process for traditional herbal medicines, the summary of the pharmacovigilance system is not required as part of the marketing authorization application. Nonetheless, MAHs are obligated to operate a pharmacovigilance system and maintain a PSMF, which must be available for submission upon request. For herbal medicines that do not fall within the scope of traditional-use registration, the requirements for operating a pharmacovigilance system, maintaining a PSMF, and submitting a summary of the pharmacovigilance system apply.
- Homeopathic Medicinal Products: Homeopathic medicinal products registered via the simplified registration procedure are exempt from the requirement to submit a summary of the pharmacovigilance system. However, MAHs must still operate a pharmacovigilance system, maintain a PSMF, and make it available upon request. For other homeopathic medicinal products falling outside the scope of simplified registration, the full requirements for pharmacovigilance system operation, PSMF maintenance, and summary submission apply.
When should a Marketing Authorization Holder prepare PSMF for EMA?
A Marketing Authorization Holder (MAH) should prepare the Pharmacovigilance System Master File (PSMF) as part of their regulatory obligations during the pre- and post-authorization phases of a medicinal product. The preparation of the Pharmacovigilance System Master File (PSMF) begins during the marketing authorization application process and continues throughout the lifecycle of the medicinal product, with ongoing maintenance and updates as necessary to ensure compliance with regulatory requirements. Here’s a breakdown:
Pre-Authorization Phase:
- During Marketing Authorization Application: The MAH should begin preparations for the PSMF during the marketing authorization application process. This includes outlining the pharmacovigilance system that will be implemented for the product and ensuring that the necessary infrastructure, personnel, and procedures are in place. The summary of the Pharmacovigilance system must be included under Module 1.8.1 of the medicinal product technical Dossier.
- Submission of PSMF Information: As part of the marketing authorization application, the MAH must provide information about the location of the PSMF and demonstrate compliance with pharmacovigilance regulations. The Pharmacovigilance System Master File (PSMF) must be readily accessible for submission upon request during the evaluation of the application.
Post-Authorization Phase
- Upon Receipt of Marketing Authorization: Once the marketing authorization is granted, the MAH must finalize and maintain the PSMF. This involves documenting the established pharmacovigilance system, including organizational structure, procedures, and risk management plans.
- Ongoing Maintenance: The MAH is responsible for continuously updating the PSMF to reflect any changes or updates to the pharmacovigilance system. This includes revisions to procedures, organizational changes, updates to risk management plans, and any other relevant information.
- Notification of Changes: Any significant changes to the pharmacovigilance system or the PSMF itself must be promptly communicated to the relevant regulatory authorities and the Qualified Person Responsible for Pharmacovigilance (QPPV).
Global Regulatory Requirements for PSMF:
- In a study done recently it is revealed that now more than 30 countries have requirements for PSMF such as Australia, GCC, India, UK, Canada, Turkey, etc.
- GCC requires EU-PSMF with some additional documents.
- India requires MAH for PSMF maintenance.
- TGA Australia requires global PSMF with Australian PSMF.
Thus, we can conclude that PSMF is an essential document in getting your drug marketed in the EU market as it gives all the information related to the pharmacovigilance of the drug.
With Artixio we can help you meet all the regulatory requirements for PSMF and help you achieve a successful market authorization for your drug product.
FAQs:
Q: What is the PSMF full form?
The full form of PSMF is Pharmacovigilance System Master File.
Q: What is the MAH full form in pharmacovigilance?
MAH stands for Marketing Authorization Holder in pharmacovigilance.
Q: Who maintains PSMF?
The Qualified Person Responsible for Pharmacovigilance (QPPV) maintains the PSMF up to date.
Q: What are some of the common challenges faced while preparing for a PSMF?
Some of challenges faced while preparing a PSMF are managing and confirming the accuracy of PSMF data, continuously updating the PSMF, meeting the global requirements, etc.
Q: What is the role of QPPV in PSMF documentation?
QPPV is responsible for monitoring the PSMF preparation and ensuring its accuracy and regulatory compliance.





